| Name of Tests Conducted | From above tests,
REVOFIL was judged as |
|---|---|
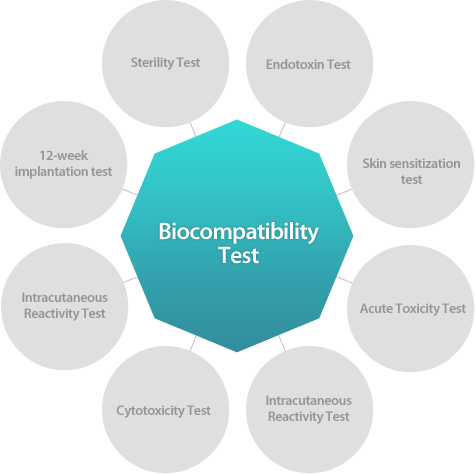
| 1.Sterility Test This test was conducted to test suitability and validation for bacteriostasis and fungistasis.in accordance with "The Korean Pharmacopoeia (9th Edition:2007)" |
Acceptable |
| 2. Endotoxin Test (LAL test)This test was conducted to detect and quantify endotoxins in REVOFIL in accordance with "The United Stated Pharmacopeia (USP 31)". <85>Bacterial Endotoxins test and "The Korean Pharmacopoeia (9th Edition:2007)" |
Acceptable |
| 3. Skin sensitization Test This test was conducted to evaluate the skin sensitizing potency of REVOFIL injection. |
Acceptable |
| 4. Acute Toxicity Test This test was performed to investigate the acute toxicity when REVOFIL injection. |
Acceptable |
| 5. Intracutaneous (intradermal) Reactivity Test This test was performed to investigate irritation potency (erythema and edema) for intradermal injection of REVOFIL. |
Acceptable |
| 6. Cytotoxicity Test This test was conducted to evaluate the potential for cytotoxicity in-vitro in accordance with International Organization for Standardization 10993: Biological Evaluation of MedicalDevices, Part 5: Tests for Cytotoxicity: in vitro Methods (ISO 10993-5). |
Acceptable |
| 7. Intracutaneous (intradermal) Reactivity Test This test was performed to investigate irritation potency (erythema and edema) for intradermal injection of REVOFIL. |
Acceptable |
| 8. 12-week Subcutaneous implantation test of REVOFIL (Injection) This test was conducted to investigate the local response (clinical sign, mortality and etc) of subcutaneous tissue in REVOFIL injection. |
Acceptable |
The results and opinions from the clinical study done for CE certification under its strict regulation for REVOFIL Fine, Plus, Ultra, Aquashine and Aquashine BR are summarized as below:
1) In most clinical cases, tissue improvement was observed in various part of skin.
2) No major side effects requiring medical attention were observed.
From the clinical
observation made in this test with REVOFIL Fine, Plus, Ultra, Aquashine and Aquashine BR and broad experience of investigator in use of similar commercially available therapy, it can be said that REVOFIL Fine, Plus, Ultra, Aquashine and Aquashine BR possesses all physical, structural and biocompatibility properties that are required of a superior gel for used in skin restoring.